
Clinical Studies
Data from observational studies and clinical trials, which depend on volunteer participants, form the foundation of regulatory authorities’ decisions to approve new therapies for broader use.

Encoded carefully designs clinical studies to assess safety and efficacy of its investigational gene therapies for the treatment of pediatric CNS disorders.
People considering enrolling their child in an Encoded study should evaluate eligibility by speaking with their child’s physician.
POLARIS
ETX101 Clinical Program for SCN1A+ Dravet Syndrome
POLARIS is the overarching program designed to optimize and accelerate clinical development of ETX101 for the treatment of people with SCN1A+ Dravet syndrome. This program includes clinical studies to assess the safety and efficacy of ETX101 in infants and young children (Phase 1/2 studies: ENDEAVOR, WAYFINDER, EXPEDITION). The POLARIS program was informed by earlier efforts to understand what patients and families need (Dravet ENGAGE), an ongoing biomarker discovery project to try and find new ways to track the disease’s progress (ELUCIDATE), and a recently completed natural history study (ENVISION), the largest longitudinal natural history study to date, which provided new important information about Dravet syndrome in young children and infants.


Clinical Studies

ENDEAVOR (NCT05419492)
A first-in-human investigational trial in the US designed to evaluate safety and efficacy of ETX101 in infants and young children with SCN1A+ Dravet syndrome

EXPEDITION (NCT06283212)
A first-in-human investigational trial in the UK designed to evaluate safety and efficacy of ETX101 in infants and young children with SCN1A+ Dravet syndrome

WAYFINDER (NCT06112275)
A first-in-human investigational trial in Australia designed to evaluate safety and efficacy of ETX101 in young children with SCN1A+ Dravet syndrome
Supporting Studies

ENVISION (NCT04537832)
An observational study of infants and children with SCN1A+ Dravet syndrome

Dravet ENGAGE
A patient-focused drug development initiative to understand the perspectives and experiences of the Dravet patient community

ELUCIDATE
A biomarker discovery program for Dravet syndrome.
More information on Clinical Studies
A clinical study is medical research involving people. There are two types of clinical studies: observational studies and clinical trials.
In an observational study, researchers observe participants on their current treatment plan and monitor health outcomes and other measures. No gene therapy or other investigational treatments are provided.
The other main type of clinical study is a clinical trial. Clinical trials test and evaluate new medical treatments or procedures in human volunteers. They are typically conducted after preliminary safety and efficacy is shown in preclinical (non-human) studies, and are the primary way that clinical researchers and regulatory agencies evaluate whether a potential new treatment or intervention is safe and effective in people. Clinical trials are governed by the FDA in the United States and by similar regulatory agencies in other countries.
Below is an overview of the traditional stages of trials for a therapy in development. However, this is just a guide: With innovative trial designs for serious and rare diseases for which there is a clear unmet medical need, phases sometimes can be combined to make the evaluation process more efficient.
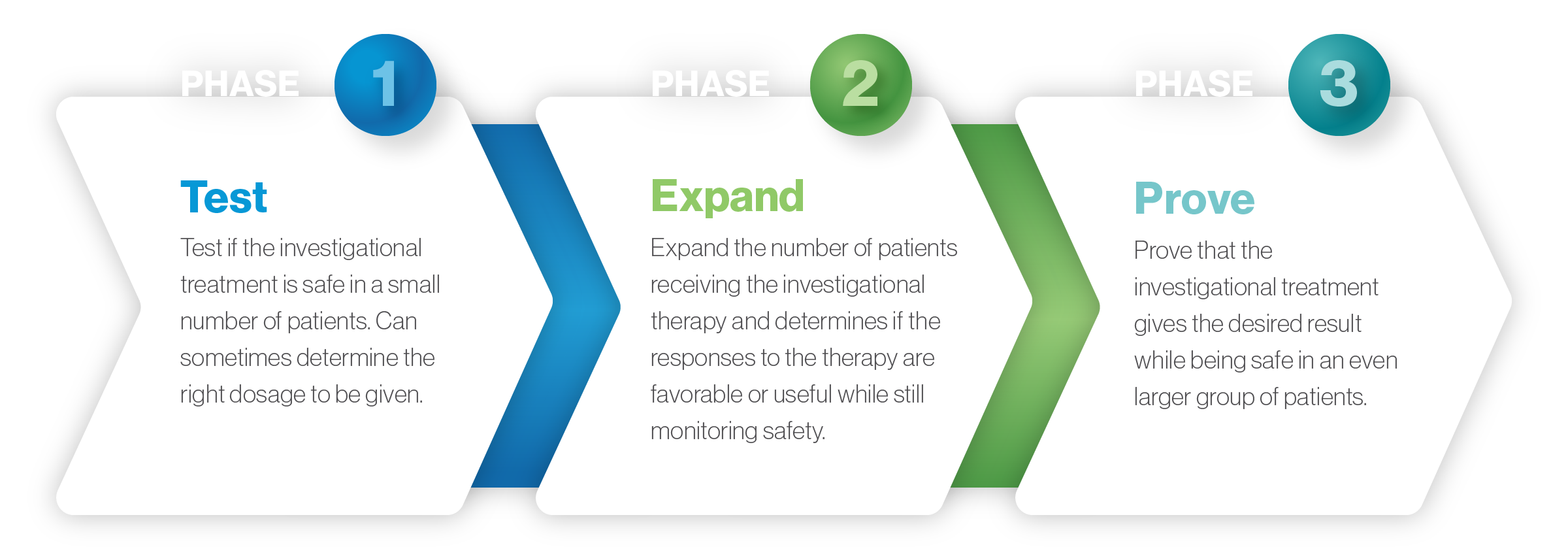
Combining phases through expedited regulatory pathways can help accelerate the assessment and approval process for therapies that meet certain criteria while still maintaining safety.
Visit these websites for more information on the process of clinical studies:
Thank you!
We would like to thank the healthcare professionals; medical, scientific and other experts; regulatory officials; and the patient community for their collaboration to design robust and meaningful clinical studies. We also are grateful to all study participants, as well as their family networks and healthcare teams, whose support enables Encoded’s clinical research to progress. Thank you!