
Genetic Medicines
Product Engine
We can take gene therapies from concept to clinic, employing our end-to-end capabilities across technology, preclinical, process sciences and manufacturing, as well as clinical development. Our ambition is to enable gene therapy to be a viable therapeutic option across multiple diseases, common and rare, in the coming years.
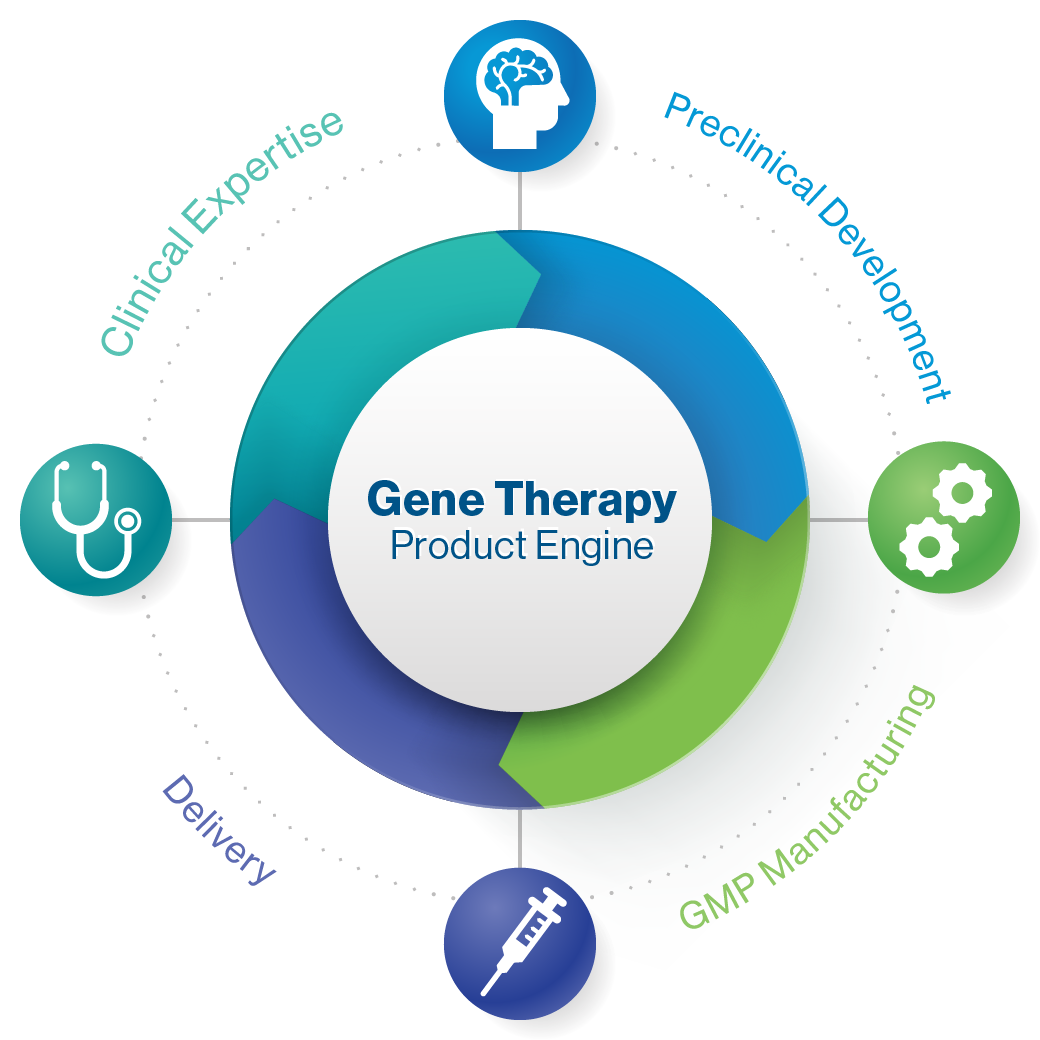
A Viable and Sustainable Gene Therapy Product Engine
Preclinical Development
Innovative therapeutic approaches that target the underlying genetic and cellular mechanism of disease for optimal safety and efficacy
GMP Manufacturing
GMP manufacturing processes, analytical tools and quality systems can be deployed across all programs
Delivery
Experience across multiple delivery modalities for indication-driven optimization of biodistribution and route of administration
Clinical Expertise
Established clinical site relationships, global regulatory expertise, and experience with innovative trial designs for pediatric CNS disorders
Encoded is independently building a pipeline of potentially best- and first-in-class therapies focused on central nervous system (CNS) disorders. Our research platform is internally developed, and our programs are wholly owned. We aim to continue to develop our pipeline utilizing our end-to-end capabilities as well as leveraging our research platform to broaden our therapeutic reach independently as well as through industry partnerships.
Applying Our Diverse Expertise to Precision Gene Therapy
We have the right combination of innovation and experience to realize the full potential of one-time gene therapies. Our leaders bring to Encoded diverse expertise across genomics, rare disease and all aspects of gene therapy development.


Best-in-Class GMP Manufacturing
Although hundreds of cell and gene therapies are currently in development, issues with supply continue to impede access and present a bottleneck for the industry. Encoded’s manufacturing ecosystem is built on the principles of flexibility, transferability and scalability.
The operational and commercial viability of gene therapy is dependent on meeting high-quality standards and yielding efficiency that can address rare and common indication supply requirements.
Encoded’s GMP manufacturing facility assures supply and cost efficiencies across a portfolio of clinical and research programs.
Encoded has invested in these capabilities since our inception and has made tremendous strides in addressing scale challenges to ensure gene therapy meets exponential yield improvements over time, consistent with earlier biologic modalities. Critical components of our ecosystem include the following:
- Internal talent who are subject matter experts on process development, analytical sciences, GMP manufacturing, supply chain and quality assurance for gene therapy
- Process Development that meets current AAV commercial-scale requirements with a clear path to exponential improvement
- Infrastructure that meets GMP-scale requirements for multiple programs
- GMP ecosystem that includes preferred CDMO relationships
